CS Tear Gas In Hong Kong And Elsewhere: Assessing The Hazards
Tear gas has been used in a number of incidents in recent months in Iraq, France, Chile, Hong Kong, and elsewhere. Numerous allegations about tear gas have been made, and misinformation, half-truths, myth, and suppositions have been passed around on social media.
Yet there is actually a wealth of information about tear gas available in the technical literature. The purpose of this post is to dig into the health and safety concerns behind tear gas usage.
What Is “Tear Gas”?
Generally, the term “tear gas” refers to a substance known as CS. CS, in turn, is a nickname for chemical substance 2-chlorobenzalmalononitrile. It is not a gas in normal situations for very long. CS is generally a solid, and is used in many forms.
CS is often used in burning grenades or canisters where the CS is combined with a burning filler and the resulting smoke suspends a cloud of particulates. In such form, CS can be an area-weapon, with clouds of fog or smoke used to clear buildings or large areas. Others dissolve CS in a solvent for spraying or bursting to suspend a cloud of particulates. CS is extremely irritating to skin and eyes, and has a distinct peppery smell. It is commonly used in military training as a simulant for much more dangerous substances and to help soldiers learn to put on their protective masks quickly.
It should be noted that there are other riot control agents that have been used, either historically, or in modern times. CA, CN, CR, OC (aka “Pepper Spray”), and various other compounds have been used at various points. There have been situations, such as in Iraq, where hexachloroethane (HC) smoke grenades have been used, in an unacceptable off-label usage, with or instead of tear gas grenades. However, there is little evidence that anything other than CS is in use in the current situation in Hong Kong.
Terminology is important. Some social media references to CS or other riot control agents as “nerve agents” or “mustard gas” are factually incorrect. A widely circulated myth alleges that because CS and pepper spray cause pain, and pain is transmitted by the nerves, these are therefore “nerve agents.” This is not, in fact, anything close to the actual definition of nerve agents. This has been discussed at length in another Bellingcat article.
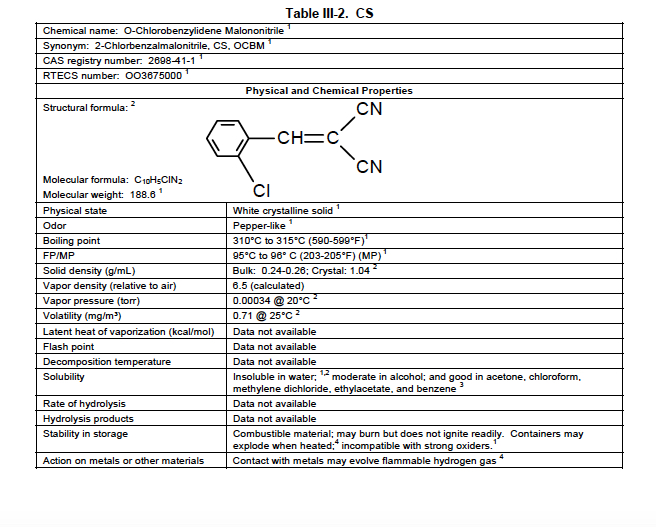
The physical characteristics of CS are shown in this table from the US Army’s Field Manual 3-11-9:
 Toxicity Of CS
Toxicity Of CS

CS has been widely considered a relatively safe substance to use because it has a very wide margin between the concentrations at which it is intolerable or incapacitating, and the concentrations at which it is potentially lethal.
Studies and references vary somewhat, but an accepted reference work (R. Gupta, Toxicology of Chemical Warfare Agents, 2nd edition, 2015, Elsevier) gives well-sourced data. CS provides irritation starting at a very low threshold of 0.004 milligrams per cubic meter of air (mg/m3).
This is an extremely small amount of material. Intolerable or incapacitating levels of CS are around 3-10 mg/m3, although individual tolerances vary significantly. Estimated lethal concentration of CS range from 25,000 to 150,000 mg/m3, over the course of one minute. This level of CS is basically impossible to achieve in any kind of field conditions.
There have been very few cases of direct lethality from CS exposure in humans, and a number of those have been due to fire and blunt trauma (from CS projectiles hitting the body), not actual CS exposure. A more comprehensive study of lethality from tear gas is here.
Vulnerable Populations
Some people are more vulnerable to CS exposure than others. CS particles are known to cause eye injury and people with contact lenses can have these particles get stuck behind the lenses, causing a higher degree of irritation than “normal.”
CS is a respiratory irritant and people with pre-existing lung conditions like asthma, emphysema, or recent smoke inhalation are likely to be more vulnerable to CS exposure. The literature on CS and asthma is inconclusive. One study has shown that women are more seriously affected by the respiratory effects of CS than men. Effects on children, pregnant women, and elderly people are poorly studied as there is no way to construct a research project on this in a way that conforms to ethical standards and there is also not enough case history from which to draw conclusions.
Four Categories Of CS Dispersal
If one examines the technical literature, not all CS is created equal, nor does every type of munition create CS with the same characteristics. The quality of CS produced by a munition appears to change depending on the method of dispersal.
Category 1: Sprays Of CS Liquid
First, there are devices that spray CS dissolved in solvents. These spray devices are commonly used by police. They are also legal for personal self-defence in many parts of the world. In the U.S., these are largely considered older technology and have been supplanted by pepper spray, which comes from chili peppers. In this category of device, the CS is dissolved in a solvent such as methylene chloride.
Category 2: Mechanical Or Explosive Dispersion Of Powder
Some devices are designed to disperse CS in powder form. One common form is the bursting grenade, of which the US M25A2 grenade is a classic example. It uses a small bursting charge to break open the grenade case and create a cloud of CS particles. Various fogger-type devices have been historically used, particularly in the Vietnam war, to make clouds of particles.
Category 3: Heat-Based Non-Munition Devices
In U.S. military training, CS grenades aren’t often used in mask confidence training. Normal practice is to heat capsules of CS powder. One study of U.S. Army CS-generation methods cited a range of 150-300° C, with an average burning temperature of 257° C. This particular study references devices used to heat and melt CS capsules in U.S. military training chambers. However, as these are used in military training in confined spaces, but not for riot control or law enforcement uses, they are not worthy of further discussion here.
Category 4: Burning CS Munitions
The largest category of CS devices are munitions that disperse the CS using a burning filler to create a smoke cloud. CS melts at 93-96° C and boils at 310-315° C.
The burning fillers, which are discussed at length below, heat up the CS, which vaporises and almost immediately condenses again, forming a smoke cloud. There is some possibility that solid CS particles also get suspended in the smoke from the burning fillers. It is this category of CS munition that poses the most hazards to health and property, for reasons that will be explained below.
Temperature Of Burning CS Munitions
Burning CS munitions will burn at various temperatures. Again, this is not an area which has received a lot of systematic study, and there’s not much reference material explaining what temperatures various CS devices reach while burning. Indeed, anecdotally, even with the same type of grenade, there will be a range of burning temperatures, as precise temperature is not one of the design specifications for such devices.
Technical literature on the burning temperature of the U.S. or other western CS grenades is sparse. The 1966 Edgewood Study helpfully asserts that U.S. grenades burn at 300 to 800° C, which is truly a wide range of results. The scientists who wrote the study replicated the contents of various CS grenades and did a significant number of test burns, with temperatures in the 500s and 600s as common results. The highest was 793° C and the lowest was 493° C. Not every test burn had temperatures measured, as the authors were looking for other things, like the percentage of CS that was effectively disseminated.
Grenades from other sources can vary significantly in burning temperature. It is anecdotally reported that the Hong Kong police have been using a new type of CS grenade that burns much hotter than previously used munitions. These new grenades are alleged to have ingredients such as magnesium and aluminium powder. A limited study using thermal imaging attempted to measure the burning temperature of some of these CS devices and the highest temperature measured was 552.6° C. However, this is well within the range of U.S. devices studied in the 1966 study.
What Is Actually In A Burning Tear Gas Shell Or Projectile?
Only a portion of the contents of a given tear gas shell will be CS. For example, a U.S. military-specification M7A3 CS grenade has about 128g of CS in pellets and about 212g of filler. A wide variety of materials appear to have been used as the burning fillers over the previous decades. These have included such materials as sugar, sulphur, magnesium carbonate, sodium bicarbonate, Fuller’s earth, kaolin, potassium chlorate, and various other components. The contents of the U.S. Army CS burning munitions are discussed the 1966 study cited above.
It should be noted that the various roles of the chemicals in the filler are usually well established. Potassium perchlorate is an oxidizer. Sugars such as sucrose or lactose work as fuels. Magnesium carbonate, sodium bicarbonate, and Fuller’s earth work as moderators, slowing the rate of combustion. It is not at all clear that every CS munition has such moderating ingredients.
CS And Human Skin
Burning CS munitions can cause burns on human skin. The majority of the cases described in medical literature are from actual direct contact with burning grenades or projectiles. This is understandable, given the temperatures discussed above.
CS particles will be hot enough to cause thermal burns for some period of time, particularly close to the actual point of discharge. Both the smoke from burning fillers and the temporarily vaporised CS emitted from a burning grenade will be hot enough to cause burns. For example, persons using oven mitts or other improvised techniques to pick up burning CS grenades can easily be exposed to CS particles and vapours hot enough to burn skin.
Accidental And Deliberate Fires
It is very important to note that even burning grenades at the low end of the range of possible temperatures, a burning CS grenade or projectile can set other things on fire. Even spent grenades may still be hot enough under some circumstances to cause fires.
CS grenades have been known to malfunction and have a condition colloquially known as a “hang-fire” wherein the ignition fuze malfunctions in some way and the grenade or projectile starts burning later than intended. Such a grenade, swept up and put into a rubbish bin or trash-pile, can cause a significant fire long after its intended use. These scenarios raise all sorts of problems, as a complex modern urban environment has many kinds of combustible materials. Plastics, synthetic textiles and upholsteries, woods that have been treated with finishes and varnishes, cleaning chemicals, and various other materials can create a bewildering array of toxic materials when combusted.
Thermal Decomposition Of CS
There is a serious problem with heating CS to the temperatures encountered in burning CS munitions. Some portion of the CS will degrade into other chemicals.
This is not something that is particularly well-known or heavily studied, but I was able to find some significant scientific literature about what happens when CS is heated to very high temperatures, such as those at the middle and high end of the burning CS grenades.
Some studies occurred in the 1960s. In the late 1990s, a U.S. Army scientist, one Timothy Kluchinsky, studied this phenomenon and wrote his 2001 PhD dissertation on the subject. He has published extensively on the topic (one example is here).
In 1960, a Porton Down study in the UK identified and measured some CS thermal degradation products. CO, CO2, Cl-, NH4, N2O, C2H2, and water were all measured in CS. A 1969 U.S. Army study burnt CS and found CO, CO2, H2O, HCl, HCN, NH3, N2O2 and C2H2 in the smoke plume.
Kluchinksy’s studies in 2000 and 2001 used gas chromatography and mass spectrometry and discovered the presence of numerous compounds in the smoke from burning-type CS canisters. These chemicals include 4-chlorobenzylidenemalononitrile (an isomer of CS), 2-chlorobenzaldehyde, 2-chlorobenzonitrile, quinoline, 2-chlorobenzylcyanide, 1,2-dicyanobenzene, 3-(2-chlorophenyl)propynenitrile, cis- and transisomers of 2-chlorocinnamonitrile, 2,2-dicyano-3-(2-chlorophenyl)oxirane, 2-chlorodihydrocinnamonitrile,benzylidenemalononitrile, cis- and trans- isomers of 2-cyanocinnamonitrile, 2-chlorobenzylmalononitrile, 3-quinoline carbonitrile, and 3-isoquinoline carbonitrile.
The health effects and toxicology of this long list of substances are not very well studied. Kluchinsky commented that many of these compounds were created by CS molecules giving up either chlorine or cyanide. He eventually did work to quantify the amount of HCl (hydrogen chloride) and HCN (hydrogen cyanide) emitted by CS under various conditions.
Decomposition Of Fillers
The subject of CS grenade fillers is not well-studied and product literature often does not specify the ingredients in CS grenade fillers, particularly products produced outside the U.S. and European Union. There is no comprehensive compendium of filling materials for CS grenades and the toxicology of burning fillers is an area that has received comparatively little study. It is possible that some fillers produce toxic materials when burnt.
It is also possible that poor manufacturing practices have accidentally incorporated unusual materials into fillers. This is not an area with a high degree of regulatory standards, and it would not surprise me to find that various trace amounts of rubbish, such as plastics and metals, even floor-sweepings, end up in low-budget CS munitions. The possible scope of harm from such scenarios is nearly impossible to estimate, as the numerous variables are unknown.
What About Cyanides?
It is clear from the literature that HCN — that is, hydrogen cyanide gas — is one of the thermal decomposition products that can be created when CS breaks down at high temperatures. However, it is difficult to construct a hypothetical scenario whereby enough hydrogen cyanide is lighter than air.
This means that, in open air, it will rise and not build up. Not only that, the HCN produced from thermal decomposition of CS will be warmer than ambient air and rise even faster than if the HCN were at the same temperature. Cases of toxic exposure to hydrogen cyanide generally occur in confined spaces. Furthermore, HCN is flammable, and many of the situations and scenarios involved include high temperatures and open flames, so it is possible that the HCN would combust.
The concentrations of HCN that will cause injury or death in humans are reported differently in different reference books. Although there have been many deaths from HCN inhalation, there have been very few where the exact concentration of HCN was known, so these figures vary greatly based on different interpretations of laboratory tests with animals. It is not possible to take measurements on inhaled HCN in rabbits or guinea pigs and then directly use those measurements as indicative of toxicity in humans, for a variety of reasons. Scientists have argued for decades over the correct way to convert numbers from animal models into human equivalents. As a result, for HCN (and actually for many other toxic inhalation hazards), the calculated human toxicity will vary a fair bit.
A standard reference book lists the following concentrations for HCN, but does not define the effects rigorously:
| Concentration in parts per million [ppm] |
Effects on humans |
| 18-36 ppm | Slight symptoms after lengthy exposure |
| 45-54 ppm | 20-60 min exposures will bring effects |
| 110-135 ppm | Dangerous after 30-60 minutes |
| 135 ppm | Lethal after 30 mins |
| 181 ppm | Lethal after 10 mins |
| 270 ppm | Rapid death |
Given the variables involved, these concentrations should be considered approximate figures at best. It should be noted that the U.S. Army lists a MUCH higher concentration as a LCt50 (lethal concentration for 50% of exposed population) for HCN, 2587 ppm in Field Manual 3-11-9. It is well within the accepted scientific literature to say that there is a ten-fold variance between estimates of concentrations which are rapidly lethal.
Full toxicological reviews of HCN are here and here.
It should also be noted that HCN can be produced by fires involving materials other than CS. Some plastics can burn, causing HCN. Once again, PVC, the prolifically available plastic, can cause HCN if burnt, as can other plastics. (Link) If HCN is encountered in the field, is it coming from CS munitions or burning plastics?
There are numerous videos on social media showing people using electrochemical HCN vapour monitors to attempt to measure concentrations of HCN in the field. Given that HCN is clearly one of the thermal decomposition products of CS, this is a reasonable finding. These sensors have operating temperature ranges, beyond which they cannot be expected to give a reasonably accurate reading. Sticking the sensor into the hot gas plume from a grenade burning at, say, 700º C is likely going to expose the electrochemical sensor to temperatures outside its operating range.
For example, the MSA Altair handheld detector (a common one, but by no means the only one) has an upper operating temperature of either +50º or +60º C. However, handheld devices have numerous cross-sensitivities. For example, HCN sensors are usually also sensitive to other chemicals. This varies widely by manufacturer. Therefore, field measurements taken quickly in the middle of a disturbance need to be kept in proper perspective and may not be accurate.
With regards to exposure scenarios in Hong Kong, it is important to keep a proper perspective. It seems exceedingly difficult to construct a scenario in open air in Hong Kong where a dangerous combination of concentration of HCN over a short period of time is received. This is due to several combined factors:
- Vapour density of HCN being lighter than air, all other factors being equal, makes HCN rise
- HCN produced by burning CS munitions will be hotter, thus even more buoyant
- The majority of incidents have occurred in the open air, thus allowing HCN to dissipate even more quickly
- Furthermore, in instances in confined spaces, conventional smoke and CS build up to irritating levels that will have an irritant effect more quickly than HCN
- Some HCN may be consumed by flames almost immediately after production
- Exposure times are short, usually a few minutes or less
- Measurements in the field may not be accurate due to inherent limitations, so they may be misleading
- To date, I have seen no case history of anyone with cyanide poisoning from inhaled HCN subsequent to CS exposure in Hong Kong. If any reader has this information, please contact me.
Tear Gas, Dioxins & ‘Dioxin-Like’ Substances
The only evidence for the presence of dioxins in Hong Kong is circumstantial. Reporter Chan Yu Hong was diagnosed with the skin condition chloracne. Chloracne is widely associated on the internet with exposure to dioxins, including an article that refers to chloracne as the “hallmark of dioxin intoxication”. A standard reference book in dermatology (Rook’s Textbook of Dermatology, volume 4) states that the following chemicals can cause chloracne:
- Polychlornapthalenes (PCNs)
- Polychlorinated biphenyls (PCBs)
- Polybrominated biphenyls (PBBs)
- Polychlorinated dibenzofurans (PCDFs)
- Polychlorinated dibenzodioxins (PCDDs)
- Tetrachloroazobenzenes
- Trifluoromethyls
- Pyrazole derivatives
It is very difficult to take one case of possible chloracne and work backwards through events and circumstances and conclude that “tear gas has dioxins” in it. Without significant additional evidence, it may be impossible to link the Chan Yu Hong case to a specific acute or chronic environmental exposure.
There are many reasons for this. First of all, the medical literature shows various differential diagnoses for chloracne, as several medical conditions look very similar. We don’t know the full extent of medical testing on Chan Yu Hong or the results of any laboratory work, nor could we publish it if we did. Medical literature also has instances of chloracne being caused by unexpected sources, including excessive tobacco smoke. Latency is an issue as well. Chloracne can appear weeks or months after exposure, making it difficult to establish the chronology of events and/or make it difficult to tie the exposure to one particular location or incident.
Even if we assume that the reporter was exposed to one of the usual sources of chloracne, there are many possible scenarios other that tear gas or tear gas by-products that could, in theory, cause the presence of one or more of the chemicals above. For example, PCNs were once widespread in insulators and coatings for wires. PCNs can be created by chlorination of naphthalene. Napthalene, in turn, has many uses, such as mothballs and even can appear in pyrotechnics.
PCBs that can cause chloracne were once widespread in electrical apparatus and carbonless copy paper, as well as many other uses. PCDFs and PCDDs are certainly worth examining as a possible cause. These can be produced by fires involving PVC plastics. A fire involving PVCs or similar plastics can cause exposure to chemicals that cause chloracne. A basic internet search shows numerous vendors in Hong Kong selling wholesale and retail quantities of PVC food wrap. Fires involving rubbish bins, bags, or containers can easily be seen in various videos and photographs from demonstrations and police activity in Hong Kong. Given such information, it is difficult to presume that a single case of chloracne is directly linked to tear gas usage. It seems more likely that a fire, possibly caused by a hot CS munition, would be the cause.
It is difficult to comment upon safe or unsafe levels of exposure the chemicals in this category. This is due to several reasons. First, there is a multiplicity of different substances in this category. Second, the depth of knowledge about the toxicology of these substances varies greatly. Third, there are differences between acute exposures to higher concentrations of substances and longer-term sub-acute and chronic exposures to lower levels. Fourth, route of exposure matters as well. Was the substance inhaled, absorbed through the skin, or ingested through food or drink? Was the food or drink contaminated, or did the person have the contaminant on their hands? Were there multiple routes of exposure? These different routes of exposure will have different toxicokinetics.
In the particular case of chloracne in Hong Kong, it is impossible to conclude that it was or was not the direct result of exposure to CS. To make such a conclusion would mean working backwards from the diagnosis of chloracne, through an unknown latent period, to either a specific acute exposure or a longer chronic exposure to one of a number of substances, which are not specifically identified to us, through a route of exposure that is not specified.
Other Toxic Materials
There are many other potentially toxic substances that could be emitted from rubbish fires. Given the complexity of the Hong Kong urban environment and the wide variety of waste materials that could be involved, it is difficult to speculate as to what other categories of toxic chemicals might be contained in smoke from such fires.
This Australian study highlights the complexity of this issue.
There are numerous other studies on the subject of waste fires easily available on Google Scholar or through libraries, such as this.
Long-Term Effects
It is difficult to speculate about long-term effects in the Hong Kong scenarios, for the simple reason that nobody really knows the answer to the question “Exposure to what chemical(s)?”
If we cannot narrow down exposure cases to a particular chemical, there is really no easy way to discuss long-term health effects. As with the chloracne case discussed above, if someone who had been exposed to a week or a month of sporadic CS doses in Hong Kong develops a particular form of cancer five years hence, there will be no credible way to retrospectively trace that cancer back to the CS exposure.
As far as long-term health effects of exposure to CS tear gas, there have been some studies of the subject. A 2017 review of studies of injuries and deaths from riot control agents looked at 31 different studies that included 5131 people and 9261 injuries (some people had multiple injuries). This study showed only 58 persons with permanent injuries or disabilities from riot control agent exposures. However, 18 of the 58 were from physical trauma from injuries caused by projectiles, not from chemical exposures. Also, the review was not exclusively devoted to injuries from CS, but included other riot control agents. In addition, some of the long-term effects were psychiatric in nature, which may or may not be directly linked to tear gas exposure, as they could also be the result of other stresses during civil disturbances.
The U.S. Army’s textbook Medical aspects of Chemical Warfare devotes several paragraphs to the subject of long-term effects of CS. This book identifies “reactive airways dysfunction syndrome” [RADS] — a condition which is similar to asthma — as a possible long-term complication after CS exposure. A 1996 study explores one of these cases. There are numerous articles and websites which explains RADS. It should be noted that there are many possible causes, including chronic exposures to irritants such as chlorine and ammonia. RADS is a very loose diagnosis with no one consistent set of diagnostic criteria. There are dissenting articles (example) in the medical literature that express the view that RADS is too vague of a diagnosis. Some sources identify a 1960 study on workers in a CS factory, but I was unable to locate a copy of this study. Any readers with access to this paper are encouraged to contact me via the comments section.
Decontamination Of CS
Decontamination of CS is actually very simple. CS is easily removed by soapy water. Furthermore, CS hydrolyses readily in water. CS particles soaked in water will be neutralised within a few hours. The rate of hydrolysis is increased if the water is made more alkaline, even by a little bit. Adding some bicarbonate of soda will increase the rate at which the CS is neutralised.
The U.S. Army textbook claims that “a solution containing 6% sodium bicarbonate, 3% sodium carbonate, and 1% benzalkonium chloride was found to bring prompt relief of symptoms and to hydrolyze CS for skin decontamination.”
As far as restoration of buildings and furnishings, this is a more complex subject. Vacuums with HEPA-quality filtration can be highly useful. This relatively old paper contains some useful advice. Health authorities in Scotland published this advice. Additional resources are online.
Conclusions
There are three interrelated conclusions to this review of technical information about CS tear gas.
Conclusion 1: Not All CS Is Created Equal
This paper identifies four different ways CS can be dispersed. The method that disperses CS through high temperature burning munitions is more dangerous than other methods due to both high temperatures and the production of thermal decomposition products.
Conclusion 2: Use Of Hot CS In A Complex Urban Environment Poses A Number Of Hazards
Use of high temperature CS munitions in large number in a complex dense urban environment such as Hong Kong poses a number of threats.
High temperature munitions can cause fires, which can cause exposure to a wide variety of exotic substances.
Conclusion 3: Injuries And Illnesses In The Hong Kong Environment May Not Be Due Directly To CS Exposure
Injuries and illnesses that are not typically found in CS tear gas exposures or the technical literature may or may not be directly attributable to CS. A variety of possibilities exist, which include, but are not limited to the following:
- Unusual CS exposures. It should be noted that the large majority of the technical literature on CS exposure is based on exposure cases of healthy adult males or laboratory animals. The “normal range” of effects is likely to be different with females, children, the elderly, and people with medical conditions that give them extra vulnerabilities (asthma, emphysema, skin conditions, eye conditions, etc.). Given the size of the affected population in Hong Kong, it is statistically extremely likely that there will be some exposure cases that provide 1 in 100, 1:1000, 1:10000, or even hitherto undocumented signs and symptoms.
- CS decomposition products. Burning CS munitions will produce byproducts, but the toxicology is poorly understood. HCN toxicity is unlikely, due to the relatively inability of warm HCN gas to build up in open air, but there are other possibilities.
- Decomposition of fillers: Poorly made CS munitions may have ingredients that are not understood. Low quality munitions may have unknown substances in them.
- Fires causing exposures: Hot CS munitions will set things on fire. There are many possibilities for toxic exposures from various products, including plastics. Some of these may pose more health hazard than any possible CS exposure. Reports of “dioxin” exposure may be more due to fires than CS exposure.
Unfortunately, working out the exposure case history of any single individual may be very difficult.
Notes: An earlier version of this article first appeared on the HK OSINT web page. Not every hyperlink gives full access to the cited sources. Contact the author by the comments section or by email at dan@kaszeta.org if you have any questions about sources and references.
